Lithium Information
Why Lithium?
Lithium-ion batteries are a dominant battery technology specifically for electric vehicles. We expect this battery technology to remain in focus for electric vehicles in the future. Lithium is a silvery-white and very soft metal from the group of alkali metals in the periodic table and the element with the lowest density. In nature, lithium occurs only in compounds due to its high reactivity. It becomes solid at room temperature and is therefore very suitable as an active material in batteries.
Lithium is used for the production of aluminum, batteries, glass and ceramics, but is also used in rocket fuel and lasers. In addition, this element is used in batteries for industrial products such as power tools (garden equipment and tools) and for large-scale energy storage. Lithium is used in more than 37% of the world’s rechargeable battery production. These include lithium-ion batteries for consumer electronics products such as smartphones, laptops, tablets and smartwatches, as well as batteries for electromobility applications such as pure e-cars, hybrid vehicles and e-bikes.
The global market for lithium batteries is expected to grow very strongly in the coming years. Rising demand for electric vehicles will drive the growth of the lithium market, as the number of new registrations for hybrid and electric vehicles powered by rechargeable lithium batteries is increasing rapidly.
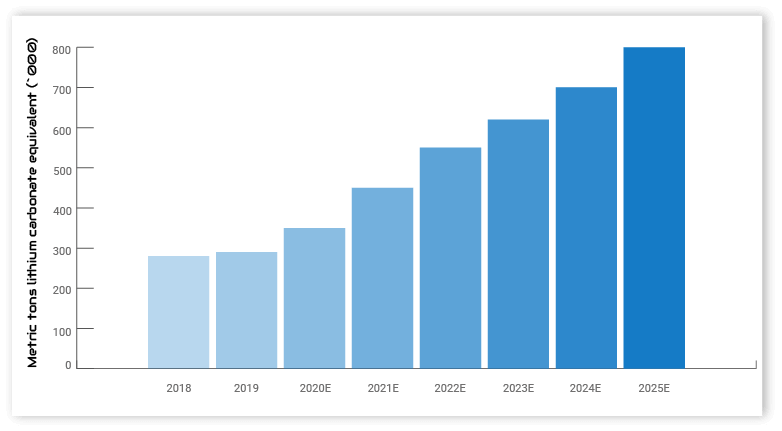
Demand for lithium will increase by 130% between 2020 and 2025. Electric vehicles accounted for approximately 39% of demand in 2020. This share is expected to increase to well over 60% by 2025. The remaining 42% is split for batteries in consumer electronics, energy storage batteries and a variety of industrial processes (s. US Geological Survey).
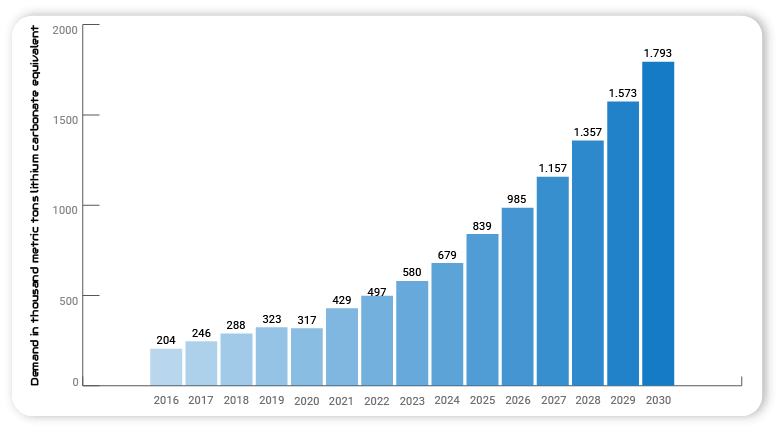
In 2030, global demand for lithium carbonate is expected to reach 1.79 million tons. Electromobility, which is becoming increasingly influential, will strongly influence the demand for batteries and thus also lithium consumption.
Where is lithium located in a battery?
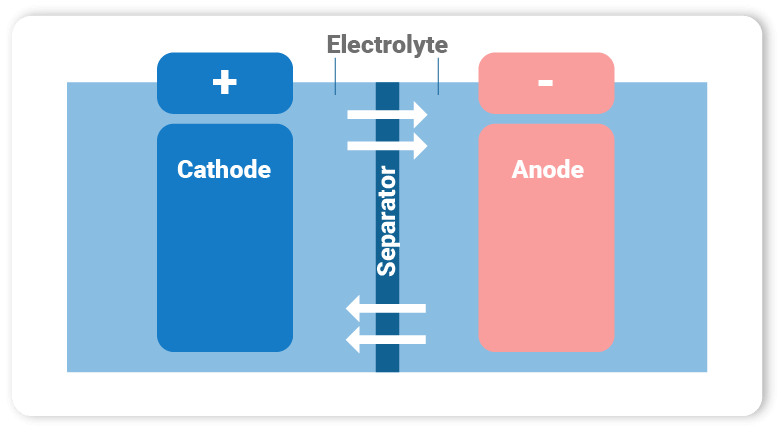
A battery consists of a cathode, an anode, a separator and the electrolyte.
The electrons flow between the anode and cathode through the electrolyte. The separator has the task of separating the electrodes from each other and enabling the transport of lithium ions, which are responsible for the current flow in the cell.
In modern batteries, lithium is contained in the cathode and in the electrolyte. Nickel-metal hydride (NiMH) batteries for electric vehicles and lithium-ion (Li-ion) batteries are considered to be at the heart of the world’s future power supply. Enormous demand for nickel-manganese-lithium-ion battery cells is also predicted for consumer electronics, electric and hybrid vehicles, and battery systems for storing electricity generated from renewable energies.
According to the current state of the art, there are 3 types of lithium-ion batteries for use in electric vehicles:
- LFP: lithium iron phosphate
- NCM: lithium nickel cobalt manganese oxide
- NCA: lithium nickel cobalt aluminum oxide
The trend among battery manufacturers indicates that the next battery technology will be a solid-state lithium-ion battery.
How is lithium produced?
Today, there are two main ways in which lithium is mined:
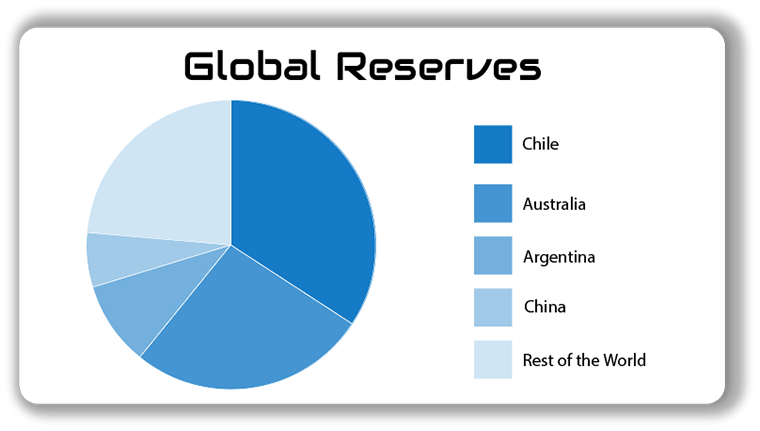
- Brines (evaporated salt lakes): Naturally occurring lithium deposits there are converted to an economically recoverable resource through evaporation. Brines account for about 60% of today’s economic lithium resources. The largest brines are located in South America (Bolivia, Chile, and Argentina) and China.
Lithium is vaporized in large tanks by solar radiation before impurities such as boron, calcium and magnesium are dissolved out. Lithium carbonate is precipitated and filtered, leaving potassium and sodium as byproducts. Brine is the most cost-effective production method due to lower energy and chemical requirements.
- Spodumene: Hard rock, particularly spodumene (lithium aluminum inosilicate), accounts for 25% of lithium resources. Spodumene must be heated and cooled again. It is then crushed and roasted with concentrated sulfuric acid. Then, sodium carbonate (soda ash) is added and the resulting lithium carbonate is crystallized, heated, filtered and dried.
There are important differences between brine and hard rock, base or precious metal projects. Brine is a fluid‐hosted form of mineralization, and thus potentially has the ability to move and mix with adjacent fluids once pumping of the brine commences.